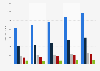
NDA/BLA filing to FDA approval times for new drugs in select disease areas 2011-2020
This statistic shows the time from filing to approval for NDA/BLA filings for new drugs in the U.S., in the period from January 1, 2011, to November 30, 2020, by disease area. It was found that new drugs in endocrine and psychiatry took the longest average time to be approved, with an average of 1.8 years.